ea of noble gases|Electron Affinity Chart (Labeled Periodic table + List) : Manila The noble gases are the elements in group 18 on the periodic table. Atoms of these elements have filled valence electron shells, making them relatively inert, colorless, odorless, monatomic gases at room temperature and . Check out all of the latest results from Portman Park, Sprintvalley and Steepledowns virtual horse racing. Shown across the UK in various high street bookmakers including Betfred, Coral, William Hill, Ladbrokes and more.
PH0 · What Are Noble Gases? Definition and Properties
PH1 · Noble gas
PH2 · Electron affinity
PH3 · Electron Affinity Chart (Labeled Periodic table + List)
PH4 · 8.14.2: Properties of Nobel Gases
PH5 · 7.5: Electron Affinities
PH6 · 22.2: Group 18
PH7 · 18.12 Occurrence, Preparation, and Properties of the Noble
Incoherent Game Cards Pdf (124) Price when purchased online. Incohearent - the Adult Party Game Where You Compete to Guess the Gibberish - by What Do You Meme? NSFW Card Game. Add $ 19 99. current price $19.99.
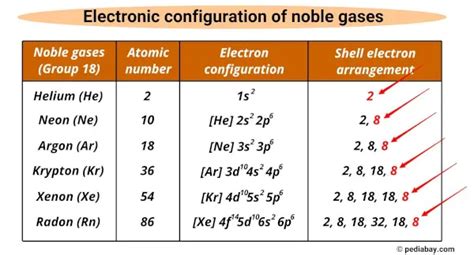
ea of noble gases*******Electron affinity is the amount of energy change (ΔE) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In other words, when the electron is added to a neutral atom, the energy is either .The noble gases (historically the inert gases, sometimes referred to as aerogens ) are the naturally occurring members of group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Under standard conditions, these elements are odorless, colorless, monatomic gases with very low chemical reactivity and cryogenic boiling points.
The electron affinity (Eea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion. X(g) + e → X (g) + energyThis differs by sign from the energy change of electron capture ionization. The electron affinity is positive when energy is released on electron capture.
The noble gases are the elements in group 18 on the periodic table. Atoms of these elements have filled valence electron shells, making them relatively inert, colorless, odorless, monatomic gases at room temperature and .The electron affinity ( EA) of an element E is defined as the energy change that occurs when an electron is added to a gaseous atom or ion: E ( g) + e − → E − ( g) energy change=EA
The noble gases (Group 18) are located in the far right of the periodic table and were previously referred to as the "inert gases" due to the fact that their filled valence shells (octets) make them extremely nonreactive.
Noble gases have uses that are derived from their other chemical properties. The very low boiling points and melting points of the noble gases make them useful in the study of matter at extremely low temperatures.
Electron Affinity Chart (Labeled Periodic table + List) The noble gases are characterized by their high ionization energies and low electron affinities. Potent oxidants are needed to oxidize the noble gases to form compounds in positive oxidation states. .The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). They earned the name “noble” because they were assumed to be nonreactive since they have .ea of noble gasesThe electron affinity (EA) for PtF 6 can be calculated as EA = -159 - 1167 + 571 = -751 kJ/mol. To put it in perspective, this is 417 kJ/mol more exothermic than the electron affinity of atomic fluorine (334 kJ). . Since then chemists have . Noble gases have uses that are derived from their other chemical properties. The very low boiling points and melting points of the noble gases make them useful in the study of matter at extremely low temperatures.The . The noble gas concentrations in the pWW were calculated from those in the mCDW and the observed WW, considering the mixed fractions of the water masses. The noble gas concentrations in the observed WW were set to the average values observed at depths of 200 – 350 m at Stations 7 and 8, where sea ice appeared to stabilize the upper water column. Step by step video, text & image solution for Which of the following are the correct statements (I) EA of noble gases is endothermic (II) EA of Fluorine is less than chlorine (III) EA of oxygen is less than sulphur (IV) EA of N is more than phosphorous The correct answer is by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.

The three heaviest noble gases react with fluorine to form fluorides. The xenon fluorides are the best characterized as the starting materials for a few other noble gas compounds. Glossary halide compound containing an anion of a group 17 element in the 1− oxidation state .The electron affinity (E ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.. X(g) + e − → X − (g) + energy. This differs by sign from the energy change of electron capture ionization. [1] The electron affinity is positive when energy is released on electron capture.
Atoms like the noble gases will not gain an electron because they are already in their most stable state with a full shell. Atoms like F will most likely gain an electron because when a free electron is added to the outer shell of fluorine, it will have obtained a full shell. Generally, atoms increasing across a period will increase in EA also. (I) EA of noble gases is endothermic (II) EA of Fluorine is less than chlorine (III) EA of oxygen is less than sulphur (IV) EA of N is more than phosphorous The correct answer is A. I,II,III and IV B. I, II and III C. II and IV D. I and IV
The seven elements—helium, neon, argon, krypton, xenon, radon, and oganesson—of Group 18 of the periodic table. All of the noble gases are present in Earth’s atmosphere and are colorless, odorless, tasteless, and nonflammable. Learn more about noble gases with this article. Binding of noble gases (NGs) is commonly considered to be the realm of highly reactive electophiles with cationic or at least non-charged character. Herein, we summarize our latest results evidencing that the incorporation of a strongly electrophilic site within a rigid cage-like anionic structure offers several advantages that facilitate the .The noble gases (Group 18) are located in the far right of the periodic table and were previously referred to as the "inert gases" due to the fact that their filled valence shells (octets) make them extremely nonreactive. The noble gases . Noble Gas Compounds. In more recent years, a number of reactions using the noble gas elements have been discovered. Although the conventional wisdom was that the complete outer shells of these elements would not allow them to react, some scientists believed that the outer electrons of the larger elements (such as Rn, Xe, and Kr) were far enough away .Noble gas analysis in sediment porewater is a robust and useful tool in environmental science that has been successfully employed to reconstruct past environmental conditions and to study diffusive transport of gases in sediments, or to identify fluxes of terrigenic helium in lake and ocean sedimen ts. However, the relatively demanding experimental effort required for noble .
ea of noble gases Electron Affinity Chart (Labeled Periodic table + List)What are the Noble Gases. The noble gases are a group of six inert (nonreactive) gases on the far right side of the periodic table. They are members of group 18, the last group on the periodic table. All of the noble gases occur in the atmosphere. In fact, air is 0.934% argon, while the other group 18 elements are present in much smaller .The elements. The Group 18 elements have a particular name Noble gases. Noble gas is translated from the German noun Edelgas, first used in 1898 by Hugo Erdmann (1862 - 1910) to indicate their extremely low level of reactivity.The noble gases were often also called the inert gases, however, since noble gas compounds are now known this name is no longer used. Noble gas alien sources may be discovered in the future. Large planets have more helium deposits than Earth. Noble Gas Properties: Key Takeaways. On the periodic table, noble gases belong to group 18, which is the right-hand column of elements. Helium, neon, argon, krypton, xenon, radon, and oganesson are the seven noble gases.Online Option. Sign in to access your institutional or personal subscription or get immediate access to your online copy - available in PDF and ePub formats
1. Introduction. The study of noble gases in seafloor hydrothermal sulfides is key to revealing the evolution of seafloor hydrothermal systems. Such studies can be used to reconstruct fluid temporal variability and to extend our knowledge of the helium/heat ratio back into the geological record (e.g., Turner and Stuart, 1992, Stuart et al., 1994a, Stuart et al., .
GIDAN ƘWARATA CHAPTER 1 BY MAMAN teddy笠 Www.bankinhausanovels.com.ng _*DEDICATION*_ _Wannan littafin tun daga farkon shi har ƙarshe sadaukarwa ce gariki Layla,Matar manya Allah ya kareki daga duk kan wani sharhi,alherin Allah ya cimmaki Sister na._ *Bamu .
ea of noble gases|Electron Affinity Chart (Labeled Periodic table + List)